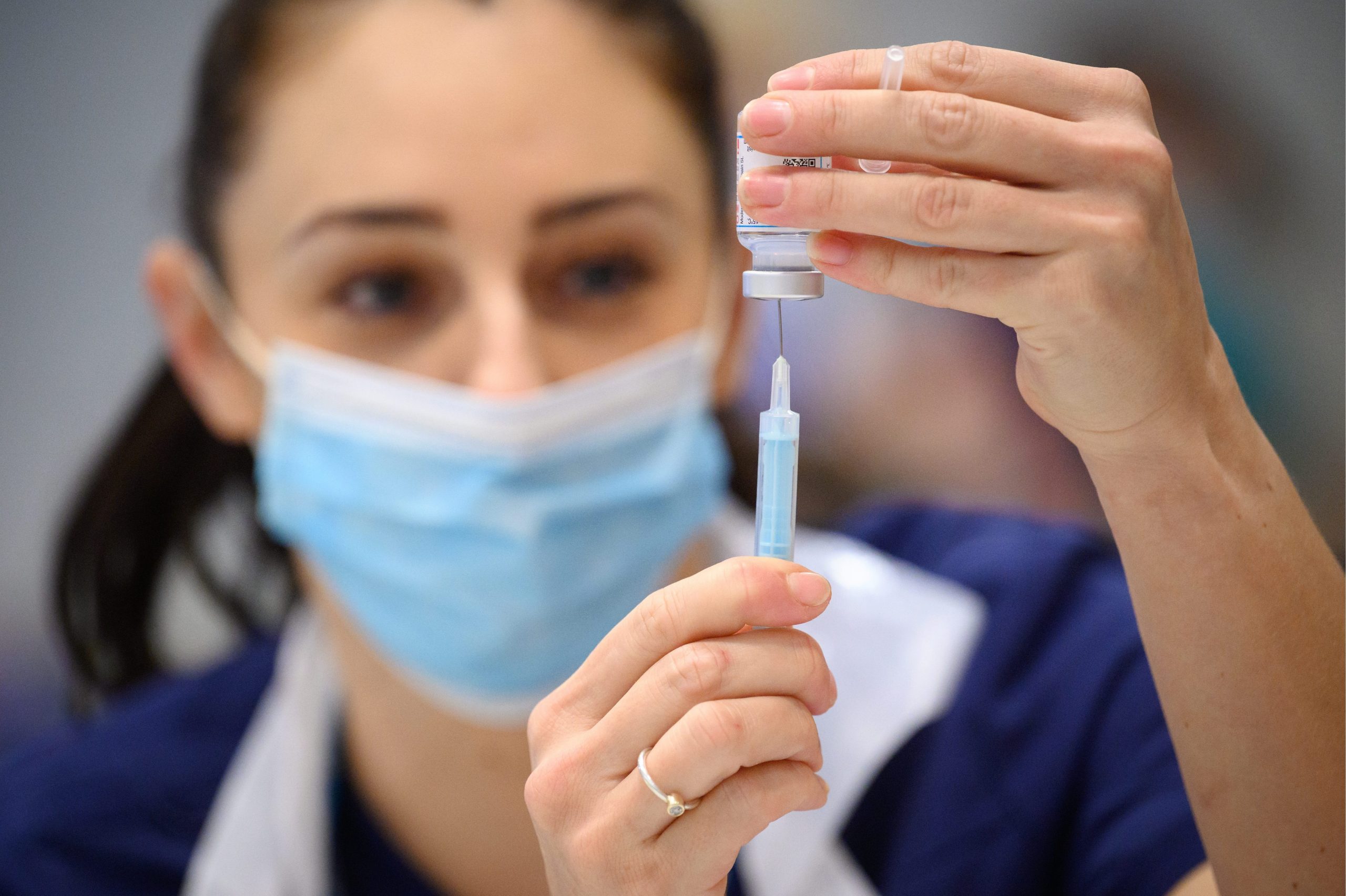
SPAIN’S new Covid-19 vaccine made by Girona-based pharmaceutical company Hipra is producing a good antibody response to the current range of Omicron variants that are behind the vast majority of new coronavirus cases in Europe.
Hipra says that 14 days after its vaccine is administered as a booster dose to people who had two Pfizer and Moderna shots, there is an increase in neutralising antibodies to the new variants.
The findings are in addition to previous results showing a higher response to the older Covid-19 strains, compared to the Pfizer vaccine.
A Hipra spokesperson said: “The vaccine is a more lasting and effective protection against new variants with a high safety profile as no adverse effects have been detected during the study phase.”
Trials will continue in regard to administering Hipra as a fourth booster dose to satisfy the demands of the European Medicines Agency which has to grant permission for the new vaccine to be used.
200 volunteers from ten hospitals in Spain will take part in new clinical trials from the end of summer.
The company believes its vaccine will act as a good alternative to existing MRNA products from Pfizer and Moderna with a ‘more sustained performance over time’.
Health Minister, Carolina Darias, stated earlier this month that fourth booster doses will start to go into arms from mid-September.
People aged 60 and other, as well as ‘vulnerable’ groups will be the first to benefit from the improved range of vaccines that counter the new Covid variants.
Darias expressed the view that subject to European Medicines Agency approval, she wanted the Hipra vaccine to be available ‘as soon as possible’.
READ MORE:
- King and Queen of Spain lead State tribute to victims of Covid-19 pandemic
- Fourth covid booster recommended for EU citizens
Click here to read more Coronavirus News from The Olive Press.