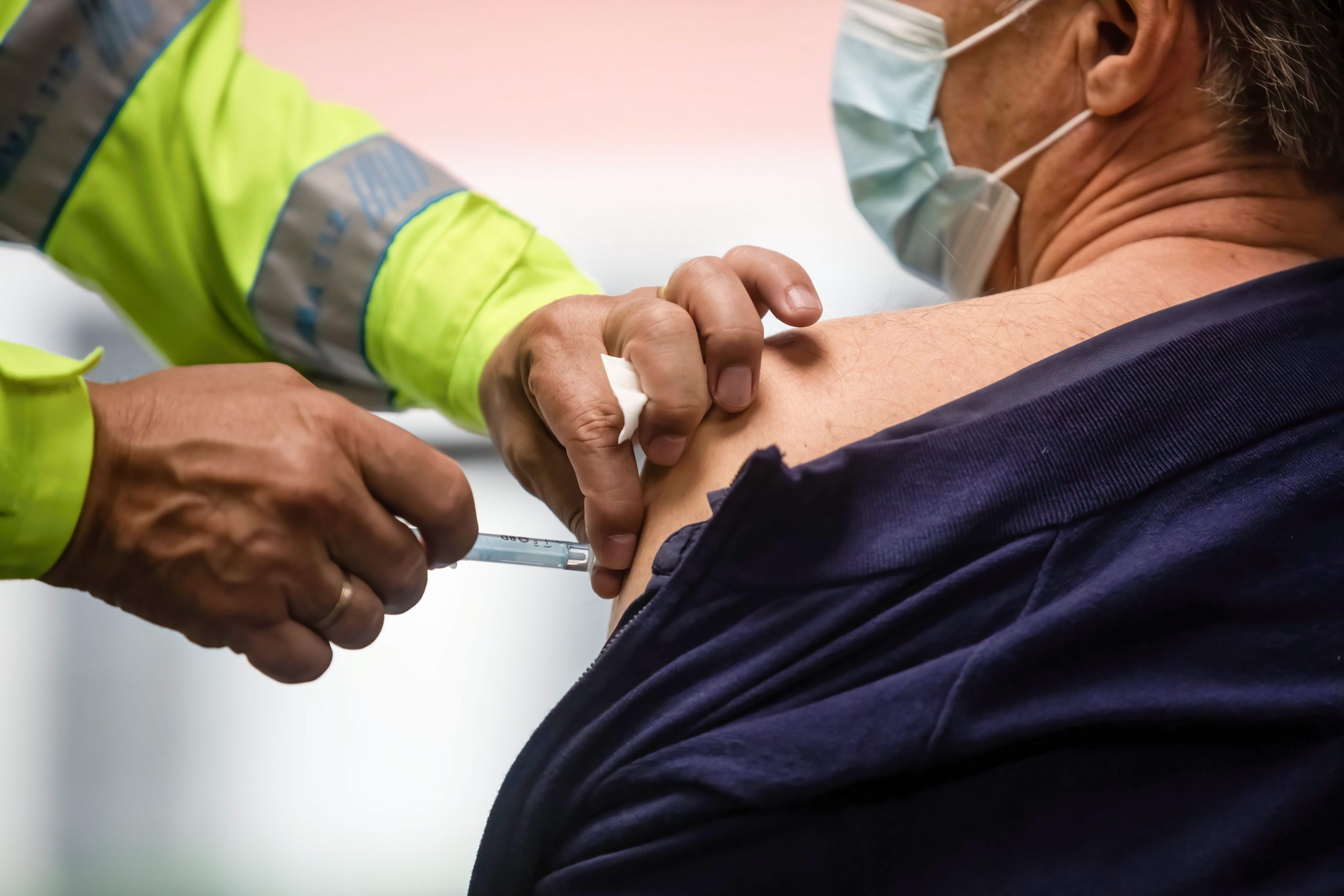
AUTHORITIES have stopped clinical trials for a Spanish-developed COVID-19 vaccine after media reports that a monkey developed a lung lesion during testing.
Over hundred volunteers were set to get the first test jabs of the new formula, but Spain’s medicines agency(AEMPS) postponed their injections due to safety concerns.
The Higher Council for Scientific Research denied suggestions that the decision was prompted by test results on the macaque monkey.
The group said that clinical trials had not been ‘cancelled’ but had been merely ‘postponed’ until the AEMPS fully evaluates the vaccine’s safety.
The vaccine produced by the National Centre for Biotechnology has been regarded as ‘promising
Work on developing the vaccine started in January 2020.
The formula was going to be tested initially on 112 people via a programme co-ordinated by Madrid’s Hospital de la Paz.
Full clinical trials would have rolled out to as many as 30,000 test subjects.
The formula has already been tested on animals like mice, hamsters and monkeys which suggested a 100% efficacy rate.
The Biotechnology Centre says they sent regular updates during its testing process to the AEMPS.
Click here to read more Coronavirus News from The Olive Press.